flickr/jakerust
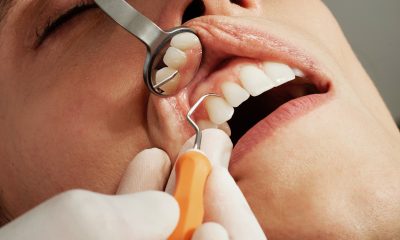
What a person wears while working tells you a lot about how dangerous their work is. In a laboratory people are typically covered from head to toe, and rarely do bare hands touch anything. It’s understandable when you consider that labs harbor a variety of highly toxic chemicals, volatile explosives and viruses that could start an epidemic.
The caliber of the staff is important at any laboratory but at all biomedical facilities security has to be a top priority. So much emphasis is put on laboratory safety that it’s easy to forget many biomedical samples are actually kept at biorepositories.
A secure biorepository is needed to store, process and deposit samples for later use. With so many delicate, yet potentially deadly materials under one roof, a biorepository has to take security and safety seriously, or we’re all at risk.
Meeting Regulatory Requirements
Regulatory requirements have been put in place to provide safety and security standards across all biorepositories. To date the FDA has created 30 guidances for Pharmaceutical Quality/Manufacturing Standards (CGMP). There is also a Code of Federal Regulations (CFR) that must be followed for recordkeeping, administrative practices, environmental protection and more.
Although these regulations have been created, some biorepositories are better at implementing and enforcing them than others. As the concern over terrorist activity and bio attacks increases, strict regulatory adherence becomes more critical.
Equipment That Protects Biomedical Samples
Biomedical sample storage requires specialized equipment that can control temperature and other environmental conditions. If the right equipment and strategies are not in place employees can become exposed to biomedical substances and/or the samples could be destroyed. Another key feature is equipment that properly secures the stored samples so that they can’t be easily accessed.
Constant Monitoring
The sensitivity of the materials requires constant monitoring. Biorepository facilities must constantly monitor the temperature of storage containers. Regular maintenance is also needed to ensure that the equipment is calibrated and operating properly at all times. All of the monitoring should be recorded so that records can be reviewed in the event there is a change or equipment failure.
Back-up Storage and Power Supplies
Because the constant operation of specialized equipment is needed, all biorepositories should have back-up storage options available as well as a backup power supply to protect the samples. In the event of a power outage facilities have a limited amount of time to get the samples moved or the power restored. Biorepositories that have backups in place are able to react quickly so that no samples are compromised.
Stringent Inventory Control
A biorepository must carefully label, process and track samples so that there is never a question about where a sample is at any time. Secured biorepositories will have a 21 CFR, Part 11 compliant system in place for recording and tracking inventory. A strict inventory system will also thwart any attempts to steal or divert samples at all stages.
Limited Access
Biorepository employees must go through a rigorous vetting process and background check to ensure that they are qualified to handle biomedical samples. The more discerning the biorepository is the better. Secured biorepositories also use activity reports to track when an employee accesses an area and anytime they handle a sample. This creates a system of accountability and ensures that no samples are moved improperly.
Plans for Containing Contamination
A contamination is a worst-case scenario for a biorepository, but it is a possibility. Containing the contamination is critical for minimizing exposure that can compromise the safety of employees and the general public. All biorepositories should have systems in place for containing contamination within the facility, especially for biological agents within classified groups 2-4 that can transfer illnesses to humans.
Without biorepositories many life-changing experiments wouldn’t be possible. When the proper protocols are followed biorepositories are completely secure facilities that provide a vital service for research and science across many industries.
Health
Experience in clinical quality: What is it, and why is it important?
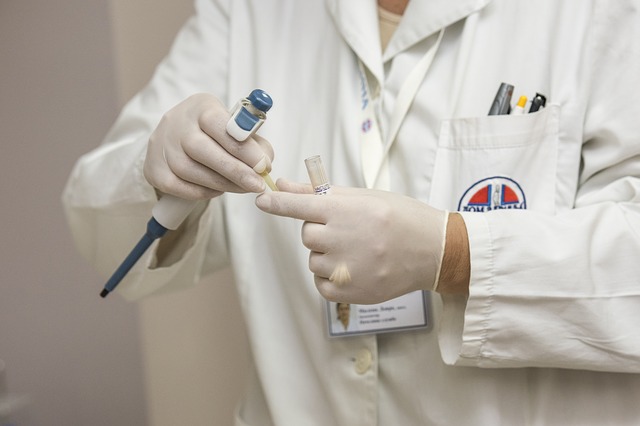
Ensuring that clinical services are of high quality and adequately meet the needs of patients is essential for any healthcare center. Therefore, they should explore all possible means to achieve these goals. Fortunately, there are specialized consulting firms that offer their knowledge and expertise to help improve the care and services provided.
Experience in clinical quality focuses on the satisfaction patients have with the care they receive at respective healthcare centers. This is crucial because it can impact the patient’s well-being, and therefore, efforts should always be made to enhance it.
What is clinical quality experience?
For a clinic or healthcare center to offer truly high-quality service, it must meet certain parameters that help patients improve their respective health conditions and feel better. Clinical quality expertise refers precisely to the quality of medical care that the patient receives, including their satisfaction and the effectiveness of the services they received. It also relates to the relationship between the patient and the healthcare provider.
This is particularly important because it can affect the health of those receiving treatments and health services. Therefore, clinics and healthcare service providers must strive to improve the patient experience as much as they can, as their patients’ health depends on it.
Fortunately, consulting agencies have specialized in this area and offer advisory services to medical practices, clinics, hospitals, and other healthcare facilities to enhance their services and optimize the clinical quality experience they provide to their patients.
How to improve the clinical quality experience?
There are several ways to improve the clinical quality experience. Firstly, it is essential for healthcare providers to listen to patients and respond to their needs. They should also be kind and compassionate and provide high-quality and effective healthcare.
Similarly, it is important for patients to actively participate in their healthcare, asking questions and expressing their concerns. They should also strictly follow the doctor’s recommendations to improve their health.
Consulting firms are crucial in achieving these objectives as they provide the necessary tools, such as cloud consultations for patients, good practices in pharmacovigilance, development of standardized operating procedures, training, and preparation for health authority inspections. They also analyze possible deficiencies, support data protection, review audit reports and corrective action plans, contributing to the improvement of healthcare processes that all healthcare centers must perform.
Why is clinical quality experience important?
Clinical quality experience is important because it can influence the health and well-being of the patient. If the patient is not satisfied with the medical care received, they are less likely to follow the doctor’s recommendations, which can lead to worsening health problems and dangerous outcomes down the line. Moreover, they are less likely to return to the doctor in the future, putting their health at risk by not having it regularly monitored.
It is essential for the clinic to have a patient experience process that develops appropriate strategies continuously and not just as an isolated action. Every healthcare center should understand the clinical, quality of life, and economic value that such strategies can contribute, recognizing that the patient experience is an ongoing interaction that involves improving communication with the patient in all aspects.
In summary, improving clinical quality experience necessitates strategies like cloud consultations, pharmacovigilance practices, and standardized operating procedures. Consulting firms contribute by analyzing deficiencies, supporting data protection, and refining healthcare processes. The significance of clinical quality experience lies in its influence on patient health, adherence to recommendations, and regular medical monitoring. Continuous improvement in patient experience ensures satisfaction, confidence in physicians, and overall health enhancement. Every healthcare center must recognize the ongoing nature of patient interaction and strategize accordingly for optimal clinical quality experience.
The clinical quality experience should always be optimized so that the patient feels satisfied with the treatments received and has confidence in their treating physicians. This way, they can better evolve their health and prevent any future ailments.
Health
The technological advances in physical and occupational physiotherapy that you should know about

Technology has reached all areas of human life to help us carry out various tasks and to make everyone’s lives easier in different ways. These advances are also in medicine and in the different therapeutic treatments that are used to improve various ailments. Learn what the most advanced methods are and how you can use them.
Health is the most precious thing for every person; extreme care must be taken to ensure the correct functioning of the body. There are many ways and procedures aimed at treating various conditions and helping to stay healthy, which have been significantly enhanced thanks to advances in technology.
The area of physiotherapy is one of those that has taken the best advantage of technological advances, and it has raised the quality and effectiveness of its therapies and procedures to levels never before experienced. Thanks to them, physical and occupational physiotherapy has improved substantially and is increasingly valued for the treatment of various health cases.
Physiotherapy programs for physical rehabilitation
Currently, there are various physical therapy software programs that help patients recover and improve their physical functionality quite efficiently. There is advanced software, with a wide range of physical exercises based on virtual reality, which are designed to promote the progressive and effective recovery of those who find themselves in the need to use them.
Digital physical rehabilitation software includes analytical and functional exercises, which can be used in the rehabilitation of neurological patients, in the recovery of musculoskeletal injuries, in the prevention of falls, in programs against premature ageing and even with children that suffer these types of ailments.
Advantages of using software in physiotherapy
Physiotherapy computer programs are health products, specially designed by professionals, specifically for clinical use. They offer many advantages, among which the following stand out:
- Enjoy the therapy sessions: the gamification that can be achieved with new technologies applied to physiotherapy turns the sessions into truly fun moments, which increases the patient’s motivation and their active participation in performing the corresponding exercises.
- Rehabilitation quantification: all kinematic parameters, such as joint ranges, measurement of the base of support, centre of gravity, number, and characteristics of steps, among others, can be consulted in detail at any time during the therapy. Additionally, they can generate detailed clinical reports on each patient, which can be printed or exported in PDF format.
- Remote sessions: technological advances have reached such high levels that they now open the possibility of applying remote sessions to the patient, thanks to the corresponding home exercise program software for physical therapy designed for this purpose. In this way, treatment can be reinforced with home sessions, which are also monitored and allow remote management, even from the centre itself. This has greatly benefited patients that have mobility problems.
Physical therapy home exercise programs are digital tools that help therapists and patients develop personalised exercise plans from the comfort of their homes. They provide a wide variety of benefits and features that improve rehabilitation and accelerate recovery.
Physiotherapists can decide with which patients and how to develop the digital physical rehabilitation exercises available to them, which can be personalised and adapted according to the needs of each patient.
Occupational therapy software programs
The occupational therapy software programs offer a multitude of resources and tools for therapists and patients, including simulations of everyday tasks, virtual activities to improve fine motor skills, time management strategies, and hand-eye coordination exercises, among others.
One of the main resources used is related to immersive virtual rehabilitation, which allows training various functions of the hand and different movements of the upper extremities that workers perform in their corresponding tasks. To do this, virtual reality and specialised programs are used that simulate environments similar to those they face on a daily basis in their jobs.
These occupational therapy software programs also include patient progress monitoring and assessment tools. They are digital solutions that improve the efficiency of occupational therapy by providing interactive virtual environments and resources tailored to the individual needs of each patient.
Personalization of rehabilitation programs
An important advantage offered by technological advances in this area is the possibility of having personalized rehabilitation programs, which therapists can use to adapt treatments to each patient’s purposes and abilities.
The personalization of rehabilitation programs substantially improves the effectiveness of treatments by addressing the unique needs of each patient. Additionally, this rehabilitation software provides useful resources to monitor and adjust as patients progress in their recovery.
This capacity for adaptation and personalization favours a firmer rehabilitation and speeds up the return to normal functionality of the treated people.
It is a feature that offers various benefits, such as the possibility of applying more individualised approaches, which guarantees that the exercises are safe, effective, and appropriate to promote recovery, and the optimization of results, as they are exercises designed specifically to meet each patient’s needs. .
They also generate greater motivation and adherence, by considering the interests, preferences, and goals of each individual treated, and help prevent additional injuries, since the exercises are adapted to the individual capabilities and limitations of each person.
In conclusion, physical and occupational therapy software has transformed the way rehabilitation is performed on people today. They are digital tools with a wide variety of features that improve the efficiency and personalization of treatments, tailoring the perfect exercise routine for each patient’s needs.
They cover various areas, from home exercise programs to creating personalized exercise plans, facilitating faster and more effective recovery for patients. But, these advances do not stop and aim to continue towards levels that cannot even be imagined, so we can count on an even more promising future in this important area of health.
Do not think about it anymore, if you are suffering from any ailment that could benefit from remote therapy, or know of someone that does, check this software today and see how your life can easily improve thanks to the help of the experts behind them. Your health will thank you.
Health
10 Tips for Starting Your Own Architectural Firm
Are you an architect with a dream of starting your own firm? If so, you’re in luck! Starting your own architectural firm can be a very rewarding experience. However, it’s not without its challenges. This blog post will discuss tips for starting your own architectural firm. With these tips, you’ll be well on your way to launching a successful business!
1) Identify Your Niche:
You’ll need to identify the type of architecture you want to specialize in before getting started. This could be anything from residential homes, commercial buildings, public spaces, or any other niche within the architectural field.
2) Build a Network:
As an architect, your network can make or break your business. Make sure to establish relationships with clients and potential partners as soon as you can. Joining local networking groups and attending events can help you build valuable connections.
3) Secure Funding:
If you don’t have enough capital to get started, consider applying for grants or loans from government agencies or investors. Having some capital behind you will give you more freedom to pursue projects that align with your goals and vision for your firm.
4) Develop a Business Plan
A successful business requires a plan. Your plan should include your vision for the firm, as well as strategies for marketing and managing finances. Having a clear roadmap to follow will make it easier to stay on track and reach your goals.
5) Protect Yourself Legally
It’s important to protect yourself legally when starting an architectural firm. Make sure you understand all of the relevant laws in your area, including licensing requirements and zoning regulations. You’ll also need liability insurance in case something goes wrong with one of your projects.
6) Invest in Technology
In order to keep up with the competition, you’ll need to invest in modern technology and tools. This could include anything from computer-aided design (CAD) software to a paper folding machine. Investing in the right technology can help you streamline processes, save time, and create a better final product.
7) Marketing
Marketing is key to the success of any business. Make sure you have a good plan in place for reaching out to potential clients and increasing your visibility. Social media, content marketing, and search engine optimization (SEO) can all be helpful in this regard.
8) Hire Talented Employees
If you want your firm to stand out from the competition, you’ll need to hire talented employees. Look for individuals with experience in the architectural field who share your vision and passion for design.
9) Develop a Brand
Creating a unique brand identity is essential if you want your firm to stand out from the crowd. This could involve creating a logo, website, or slogan that captures the essence of what your firm stands for.
10) Stay Flexible
The architecture industry is constantly changing and evolving, so it’s important to stay flexible and adapt to new trends. Don’t be afraid to try something different or take risks when necessary. Doing so can lead to greater success in the long run.
Starting your own architectural firm can be a rewarding experience, but it requires planning and dedication. Make sure to follow these tips when getting started to ensure that you set yourself up for success!
-

 Business12 months ago
Business12 months agoHow To Future-Proof Your Business With The Right Tools
-

 Travel10 months ago
Travel10 months agoTravelling from San Antonio to Guadalajara
-

 Travel7 months ago
Travel7 months agoTravel wellness tips for a healthier and more enjoyable journey
-

 Europe6 months ago
Europe6 months agoRecent Books by Boaventura de Sousa Santos: Law, Colonialism, and the Future of Europe